UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM
CURRENT REPORT
Pursuant to Section 13 or 15(d)
of the Securities Exchange Act of 1934
Date of Report (Date of earliest event reported):
(Exact name of registrant as specified in its charter)
| |
|
| ||
| (State or other jurisdiction of incorporation) |
(Commission File Number) |
(I.R.S. Employer Identification No.) |
(Address of principal executive offices) (Zip Code)
(Registrant’s telephone number, including area code)
N/A
(Former name or former address, if changed since last report)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
| |
Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
| |
Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
| |
Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
| |
Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Securities registered pursuant to 12(b) of the Act:
| Title of each class |
Trading Symbol(s) |
Name of each exchange on which registered | ||
| |
|
|
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 or Rule 12b-2 of the Securities Exchange Act of 1934 (§240.12b-2 of this chapter).
Emerging growth company
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
| Item 7.01 | Regulation FD Disclosure. |
On January 14, 2020, Helen Sabzevari, PhD, President and CEO of Intrexon Corporation, delivered the presentation attached to this current report as Exhibit 99.1 at the 2020 J.P. Morgan Healthcare Conference.
As provided in General Instruction B.2 of Form 8-K, the information in this Item 7.01 and the exhibit furnished hereunder will not be deemed to be “filed” for purposes of Section 18 of the Securities Exchange Act of 1934, as amended, nor will they be deemed to be incorporated by reference in any filing under the Securities Act of 1933, as amended, except as will be expressly set forth by specific reference in such a filing.
| Item 9.01 | Financial Statements and Exhibits. |
(d) Exhibits.
| Exhibit No. |
Description | |||
| 99.1 |
||||
| 104 |
Cover Page Interactive Data File (embedded within the Inline XBRL document) | |||
1
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
| Intrexon Corporation | ||
| By: |
/s/ Donald P. Lehr | |
| Donald P. Lehr | ||
| Chief Legal Officer | ||
Dated: January 14, 2020

Precigen, Inc. Helen Sabzevari, PhD President & CEO 14 January 2020 38th Annual J.P. Morgan Healthcare Conference Exhibit 99.1

Forward-looking statements Precigen, Inc. is a subsidiary of Intrexon Corporation (Nasdaq: XON). Some of the statements made in this presentation are forward-looking statements made pursuant to the safe harbor provisions of the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based upon Intrexon's and Precigen's current expectations and projections about future events and generally relate to plans, objectives and expectations for the development of Precigen's business and can be identified by forward-looking words such as “may,” “will,” “potential,” “expect,” “believe,” “anticipate,” “intend,” “continue,” “opportunity,” “groundwork,” “poised,” “future,” “update” and similar expressions. Examples of forward-looking statements in his presentation, include statements about the timing, pace and progress of preclinical and clinical trials and discovery programs, potential benefits of platforms and product candidates including in comparison to competitive platforms and products, and the expected closing date of transactions with Third Security, the renaming of Intrexon Corporation to Precigen, Inc., and future plans for the company’s remaining non-healthcare assets. Although management believes that the plans, objectives and results reflected in or suggested by these forward-looking statements are reasonable, all forward-looking statements involve risks and uncertainties and actual future results may be materially different from the plans, objectives and expectations expressed in this presentation. These risks and uncertainties include, but are not limited to, (i) the fulfillment of closing conditions, (ii) the distraction of management from business operations, (iii) the risks associated with separating businesses out from ongoing operations, (iv) Intrexon’s strategy and overall approach to its business model, its efforts to realign its business, and its ability to exercise more control and ownership over the development process and commercialization path; (v) the ability to successfully enter new markets or develop additional products, including the expected timing and results of investigational studies and preclinical and clinical trials, whether with its collaborators or independently; (vi) the ability to successfully enter into optimal strategic relationships with its subsidiaries and operating companies that it may form in the future; (vii) the ability to hold or generate significant operating capital, including through partnering, asset sales and operating cost reductions; (viii) actual or anticipated variations in operating results; (ix) actual or anticipated fluctuations in competitors’ or collaborators’ operating results or changes in their respective growth rates; (x) cash position; (xi) market conditions in the company’s industry; (xii) the volatility of Intrexon’s stock price; (xiii) the ability, and the ability of collaborators, to protect Intrexon’s intellectual property and other proprietary rights and technologies; (xiv) the ability, and the ability of collaborators, to adapt to changes in laws or regulations and policies; (xv) outcomes of pending and future litigation; (xvi) the rate and degree of market acceptance of any products developed by Intrexon, its subsidiaries, collaborations or joint ventures; (xvii) the ability to retain and recruit key personnel; (xviii) expectations related to the use of proceeds from public offerings and other financing efforts; (xix) estimates regarding expenses, future revenue, capital requirements and needs for additional financing; (xx) the successful completion of certain anticipated transactions, and (xxi) the challenges inherent in leadership transitions. For a discussion of other risks and uncertainties, and other important factors, any of which could cause actual results to differ from those contained in the forward-looking statements, see the section entitled "Risk Factors" in Intrexon's Annual Report on Form 10-K, as well as discussions of potential risks, uncertainties, and other important factors in Intrexon's subsequent filings with the Securities and Exchange Commission. All information in this presentation is as of the date its cover page, and Intrexon undertakes no duty to update this information unless required by law. All of the pharmaceutical products described in this presentation are investigational new drugs, which are currently undergoing pre-clinical and/or human clinical trial testing. As a result, none of them have had their safety or efficacy established or are approved by the U.S. Food and Drug Administration or any other regulatory agency. © 2020 Precigen, Inc. All rights reserved.

Intrexon: Becoming dedicated healthcare company operating as Precigen Announced January 2, 2020 Name to be Precigen with expected stock symbol of ‘PGEN’ Assets to encompass wholly-owned subsidiaries Precigen, ActoBio Therapeutics, Exemplar Genetics and majority ownership interest in Triple-Gene Increased Focus on Healthcare Dr. Helen Sabzevari appointed President and CEO of new Precigen Randal J. Kirk appointed Executive Chairman Leadership Certain non-healthcare assets* to be sold to Third Security (expected to close late Jan 2020) Interest in EnviroFlight, LLC sold to Darling Ingredients, Inc. Divestment of Non-Healthcare Assets Previous cash position and expected proceeds from divestments and stock purchase approximate $175M at year end Significant runway with increased focus and reduced cash burn for efficient use of capital Financial Strength *Ag Biotech Division (AgBio), Intrexon Laboratories Hungary (ILH), Intrexon Produce Holdings, Inc. (owner of Okanagan Specialty Fruits), Intrexon UK Holdings, Inc. (owner of Oxitec, Ltd.), Intrexon's nominal equity interests in Oragenics and Parallel (formerly Surterra), and the internet domain name DNA.com.

Precigen: Maximizing platform technology utilization Shared focus on immuno-oncology, infectious disease, cardiovascular disease, and autoimmune disorders Microbe-based agents that deliver disease-modifying therapeutics Multiple clinical and preclinical candidates Key interim data in 2020 Next generation gene and cellular therapies using precision technology Multiple clinical and preclinical candidates Initial Phase 1 data readout in 2H20 Multigenic gene therapies focused on cardiovascular disease Key asset in Phase 1 Additional Phase 1 data in 2020 Market leader in genetically engineered MiniSwine models of human disease Potential for regenerative medicine applications ‡Wholly owned subsidiary Precigen, Inc. will be renamed in connection with parent company name change. ‡
FISCAL STRENGTH Significant cash runway to deliver value inflection RAPID EXECUTION Focus on rapid execution of priority programs with the highest probability of success Precigen’s strategic objectives allow us to deliver on our vision for patients ACTIVE PORTFOLIO MANAGEMENT Continuous evaluation of portfolio based on data to make rapid go/no go decisions STRATEGIC PARTNERSHIPS Seek strategic partnerships to maximize value generation PRECIGEN’S VISION FOR PATIENTS Develop life-saving and cost-conscious therapies utilizing our cutting-edge platform technologies for patients with unmet need

CONTROL gene expression and regulation to drive safety CONSTRUCT powerful gene programs to drive efficacy DELIVER gene programs via viral, non-viral, and microbe- based approaches to drive lower costs Precigen’s technology platforms provide a strong foundation to realize core promise of precision medicine UltraVector® mbIL15 Sleeping Beauty system AttSiteTM recombinases AdenoVerseTM RheoSwitch® Kill switches Tissue specific promoters Lactococcus lactis

PRODUCT PLATFORM INDICATION DISCOVERY PRECLINICAL PHASE 1 PHASE 2 PHASE 3 STATUS / MILESTONES PARTNER PRECIGEN AG019 ActoBiotics Type 1 Diabetes Interim data in 3Q20 PRGN-3005 UltraCAR-T Ovarian Cancer Initial data in 2H20 PRGN-3006 UltraCAR-T AML, MDS Initial data in 2H20 INXN-4001 Non-viral UltraVector Heart Failure Phase 1 data in 2020 PARTNERED FCX-007 Fibroblast Cell Therapy RDEB Pivotal Phase 3 initiated AG013 ActoBiotics Oral Mucositis Phase 2 interim data in 1H20 CGF166 Gene Therapy Hearing Loss Phase 1/2 ongoing FCX-013 Fibroblast Cell Therapy Localized Scleroderma Phase 1/2 is enrolling Precigen has robust clinical pipeline of internal and partnered programs with important data readouts in 2020
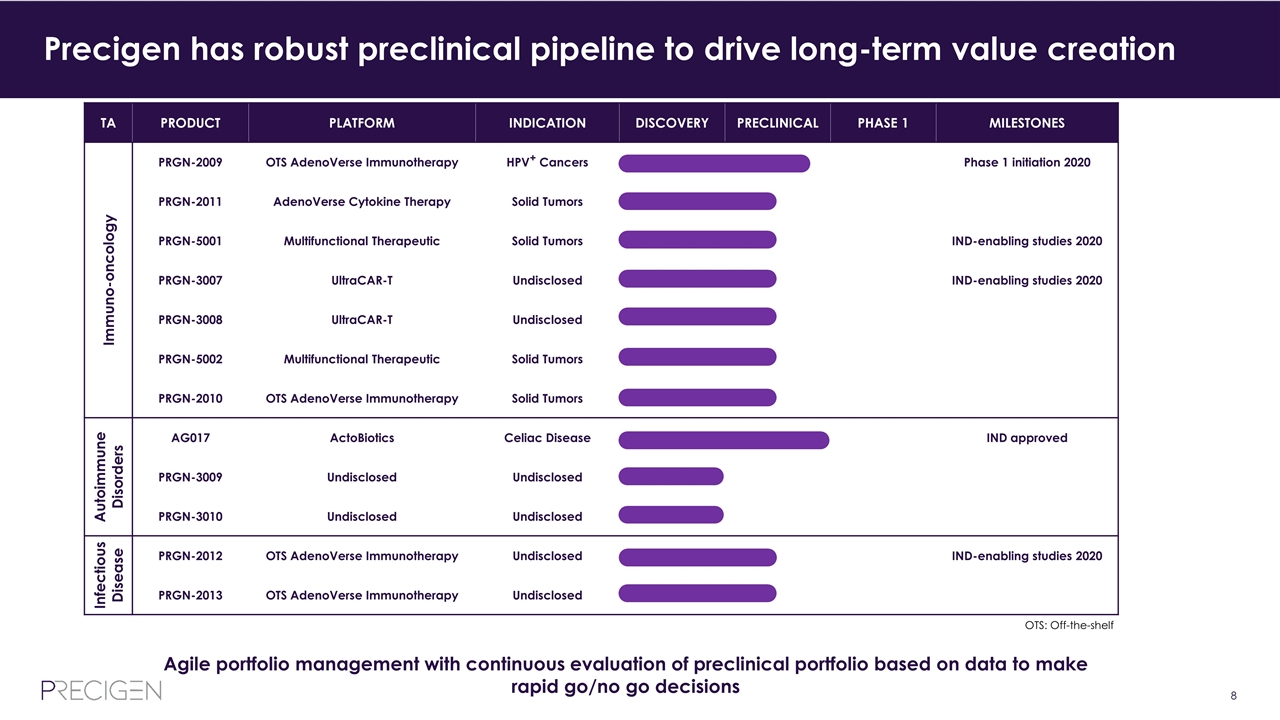
TA PRODUCT PLATFORM INDICATION DISCOVERY PRECLINICAL PHASE 1 MILESTONES Immuno-oncology PRGN-2009 OTS AdenoVerse Immunotherapy HPV+ Cancers Phase 1 initiation 2020 PRGN-2011 AdenoVerse Cytokine Therapy Solid Tumors PRGN-5001 Multifunctional Therapeutic Solid Tumors IND-enabling studies 2020 PRGN-3007 UltraCAR-T Undisclosed IND-enabling studies 2020 PRGN-3008 UltraCAR-T Undisclosed PRGN-5002 Multifunctional Therapeutic Solid Tumors PRGN-2010 OTS AdenoVerse Immunotherapy Solid Tumors Autoimmune Disorders AG017 ActoBiotics Celiac Disease IND approved PRGN-3009 Undisclosed Undisclosed PRGN-3010 Undisclosed Undisclosed Infectious Disease PRGN-2012 OTS AdenoVerse Immunotherapy Undisclosed IND-enabling studies 2020 PRGN-2013 OTS AdenoVerse Immunotherapy Undisclosed Precigen has robust preclinical pipeline to drive long-term value creation Agile portfolio management with continuous evaluation of preclinical portfolio based on data to make rapid go/no go decisions OTS: Off-the-shelf

Disrupting the market: Precigen’s UltraCAR-T™ treatment is delivered to patients one day after non-viral gene transfer Reliance on viral vectors Complexity of manufacturing viral vectors Long and complex CAR-T cell manufacturing process Long delays for patients High cost of manufacturing Exhausted T cell phenotype Major challenges in solid tumor treatment Non-viral gene delivery Simplified manufacturing of Plasmid DNA Overnight UltraCAR-T manufacturing process No ex vivo expansion necessary Reduced manufacturing cost Stem-like memory T cell phenotype Enhanced potential for expansion and persistence Conventional CAR-T Viral vectors and ex vivo expansion result in long delays for patient treatment and high cost UltraCAR-T™ Overnight non-viral gene transfer eliminates long delays for patient treatment and lower manufacturing cost 1 2 5 6 7 8 3 4 Modified from Iyer et al., Front. Med.,2018

PRGN-3005 UltraCAR-T™, a first-in-class therapy in ovarian cancer Target & Patient Population Preferentially targets MUC16+ cancer cells MUC16 overexpressed on >80% of ovarian tumors Limited expression found in healthy tissues Initial target is advanced stage platinum resistant ovarian cancer 300k diagnosed annually1/22k in US2 Phase 1 Clinical Trial Ongoing Second cohort for IP arm enrolling patients 100% manufacturing success to date Initial data readout from IP arm expected in 2H20 Encouraging preliminary findings of UltraCAR-T kinetics 1World Health Organization, International Agency for Research on Cancer, Global Cancer Observatory. Cancer Today, Estimated number of new cases in 2018, worldwide, both sexes, all ages. Accessed December 2018 via WHO IARC GCO website. 2American Cancer Society Ovarian Cancer Special Section. Access December 2018 via ACS website. MUC16 CAR Arm A: Intraperitoneal (IP) infusion Arm B: Intravenous (IV) infusion Clinical Trial Schema Advanced non-viral Sleeping Beauty system to co-express MUC16 CAR, mbIL15 and kill switch

PRGN-3006 UltraCAR-T™, a first-in-class therapy in AML Target & Patient Population CD33 is overexpressed on myeloid leukemia cells and leukemic stem cells 85-90% of AML patients show expression of CD33 on blasts 20k diagnosed in US in 20181 with relapsed or refractory AML Higher risk myelodysplastic syndrome (MDS) has US incidence >10k per year2 Phase 1/1b Clinical Trial Ongoing Second cohort of no lymphodepletion arm and first cohort of lymphodepletion arm are enrolling patients 100% manufacturing success to date Initial data readout expected in 2H20 Orphan Drug Designation recently granted by FDA Encouraging preliminary findings of UltraCAR-T kinetics 1American Cancer Society. Key Statistics for Acute Myeloid Leukemia (AML). Accessed December 2018 via ACS website. 2American Cancer Society. Key Statistics for Myelodysplastic Syndromes. Accessed December 2018 via ACS website. Clinical Trial Schema Arm 1: No lymphodepletion prior to UltraCAR-T infusion Arm 2: Lymphodepletion prior to UltraCAR-T infusion CD33 CAR Advanced non-viral Sleeping Beauty system to co-express CD33 CAR, mbIL15 and kill switch

PRGN-2009 off-the-shelf AdenoVerse™ immunotherapy for HPV+ cancers Target & Patient Population Designed to activate immune system to recognize and target HPV+ solid tumors HPV+ cancers represent significant health burden in head and neck, cervical, vaginal and anal cancer Gorilla adenoviral vector with large payload capacity and ability for repeat injections Optimized HPV antigen design for improved immune response differentiates from competition Phase 1 Clinical Trial Initiation Upcoming Currently under development through CRADA with Dr. Jeffrey Schlom at NCI Phase 1 clinical trial initiation expected in 2020 PRGN-2009 immunotherapy effectively controls tumor in murine model of HPV+ head & neck cancer PRGN-2009 Control Vehicle Gorilla adenovector with novel HPV antigen design PRGN-2009 Control CD8+ T cells / mg tumor Anti-tumor response CD8+ T cell infiltration in to tumor

Multifunctional therapeutics overcome tumor microenvironment immunosuppression and improve T cell function compared to anti-PD1 in preclinical mouse models Multifunctional Therapeutic Platform Simultaneously targets multiple pathways to address senescence and trafficking of T lymphocytes in tumor microenvironment Exhibited superior anti-tumor effects compared to anti-PD1 mAbs Data supports potential for expansion to multiple targets Initiate IND enabling studies for PRGN-5001 in 2020 Evaluating the optimal path forward for Multifunctional Therapeutic Platform including ongoing partnership discussions with multiple companies PRGN-5001 exhibits superior anti-tumor effect compared to anti-PD1 in humanized mouse model of lung cancer PRGN-5002 exhibits superior anti-tumor effect compared to anti-PD1 in humanized mouse model of cervical cancer

AG013 for oral mucositis (OM) AG013 is oral solution of ActoBiotics™ to deliver human Trefoil Factor 1 (hTFF1) to mucosal tissues Ease of administration Target & Patient Population OM is a side effect of chemo/radiation therapy in patients treated for head & neck cancer and other solid tumors No drug is approved to prevent OM in the broad cancer population 2019 addressable population: approximately 850k† Phase 2 Clinical Trial Ongoing in Head & Neck Cancer Patients Enrollment completed in 4Q19 Interim data from Phase 2 expected in 1H20 Orphan Drug status in European Union FDA Fast Track designation Development under partnership with Oragenics AG013 delivers hTFF1 via genetically modified L. lactis The bacteria is freeze dried into vials Patient mixes powder with a raspberry-flavored solution Patient swishes for 30 seconds after every meal The activity delivers a protein called trefoil factor, which regrows the oral lining 1 2 3 4 5 † Sources: http://oncolex.org/Head-and-Neck-cancer/Diagnoses/Oral-cavity/Background/Prognosis https://www.uptodate.com/contents/epidemiology-and-risk-factors-for-head-and-neck-cancer https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/cancer-treatment-and-survivorship-facts-and-figures/cancer-treatment-and-survivorship-facts-and-figures-2014-2015.pdf http://www.onclive.com/publications/oncology-live/2014/july-2014/study-finds-mouth-rinse-alleviates-oral-mucositis-symptoms-in-head-and-neck-cancers https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3662500/

AG013 Phase 1b data: Demonstrated efficacy vs placebo * Ulcerative Oral Mucositis (UOM) : WHO score ≥ 2 Limaye et al., 2013, Cancer Clinical Trial Design Phase 1b single blind, placebo-controlled study; 6 centers in the US Study measured safety and tolerability of locally-applied AG013 in head and neck cancer patients receiving induction chemotherapy Clinical Trial Data Summary Safe and well tolerated Consistent effect of active versus placebo across all dose frequencies without a dose frequency-dependent effect 29% of responders reported fewer than 2 days of UOM while all placebo-treated patients experienced more than two days of UOM 40% reduction in unscheduled office and emergency room visits compared to placebo 35% reduction in percentage of days with UOM compared to placebo AG013 reduces cumulative days with UOM* vs placebo (PBO) by 35% AG013 treatment reduced unplanned office & ER visits 29% of responders had ≤1 day of UOM*

AG019, a first-in-class therapy for type 1 diabetes (T1D) Target & Patient Population First-in-class disease modifying antigen-specific immunotherapy to prevent, delay or reverse T1D Recent-onset T1D patients (children and adults) with residual functional Beta-cell mass Phase 1b/2a Clinical Trial Ongoing Phase 1b/2a study to assess the safety and tolerability of different doses of AG019 administered alone (Phase 1b) or in combination with teplizumab (anti-CD3 mAb) (Phase 2a) Enrollment and treatment completed in Phase 1b (monotherapy); No discontinuation in treatment to date Enrollment ongoing in Phase 2a (combination) cohorts; No safety issues to date Interim data readout expected in 3Q20 AG019 is a capsule formulation of ActoBiotics™ to express human Proinsulin (hPINS) and human Interleukin-10 (hIL-10) AG019 + anti-CD3 treatment is highly effective in diabetic mice Co-expression of KI67 and FoxP3 reveal local proliferation Takiishi et al., 2012, JCI NOD Recent Diabetic 89% of new-onset diabetic mice cured by AG019 + low dose anti-CD3 PINS-specific FoxP3+ Treg cells accumulate and proliferate in the pancreas & peripheral lymph nodes hPINS & hIL-10 genes inserted

INXN-4001, novel gene therapy product for heart failure (HF) Target & Patient Population Heart failure is a complex, multi-modal and progressive disease that requires targeting multiple pathways for successful outcome Therapeutic options for end-stage HF are limited Three effector genes in INXN-4001 designed to address multiple malfunctions of cardiomyocytes in patients with heart failure Approximately 6M adults in US have heart failure Phase 1 Clinical Trial Ongoing Phase 1 enrollment complete Initial data shows improvement in cardiac function and no product related adverse events Phase 1 data completion in 2020 Retrograde Coronary Sinus Infusion (RCSI) Avoids wall thickness concerns, potential coronary dissection, potential ventricular perforation, electro-mapping and thrombus formation Is on the low pressure, venous side Allows for much larger dose delivery to entire ventricle Non-viral triple effector plasmid based on UltraVector® platform to simultaneously express human S100A1, SDF-1α, and VEGF-165
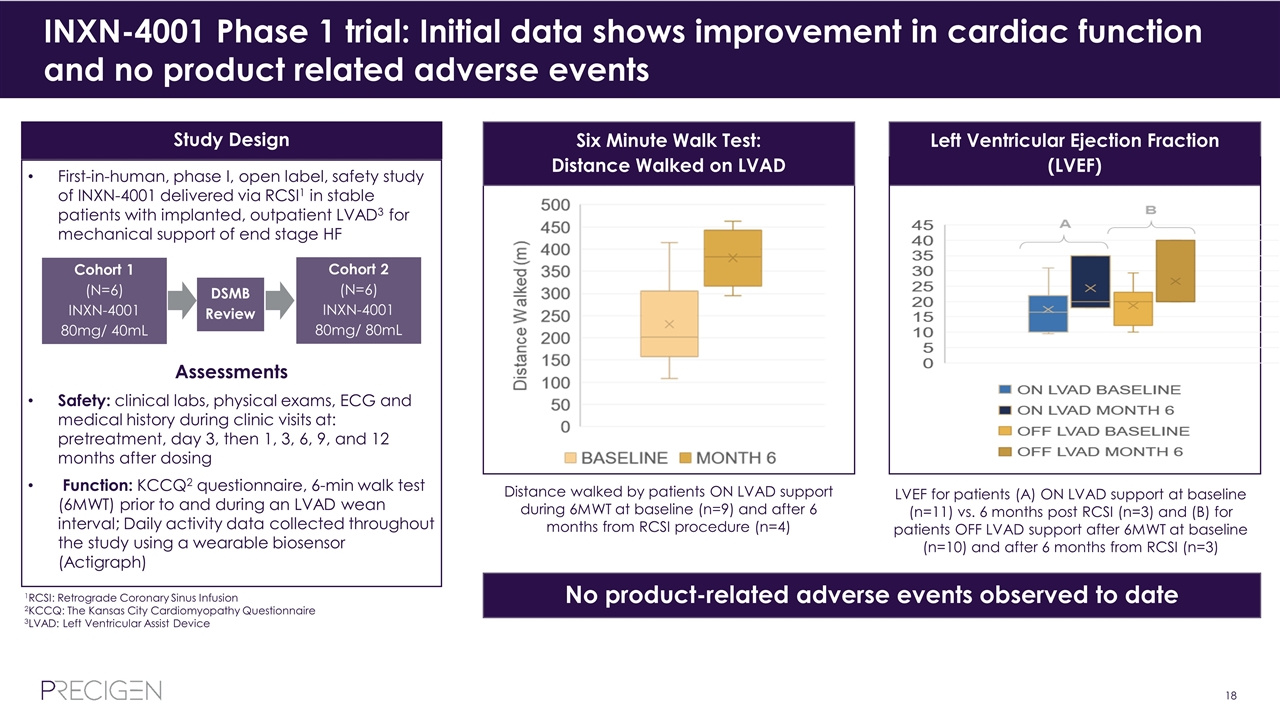
INXN-4001 Phase 1 trial: Initial data shows improvement in cardiac function and no product related adverse events First-in-human, phase I, open label, safety study of INXN-4001 delivered via RCSI1 in stable patients with implanted, outpatient LVAD3 for mechanical support of end stage HF Assessments Safety: clinical labs, physical exams, ECG and medical history during clinic visits at: pretreatment, day 3, then 1, 3, 6, 9, and 12 months after dosing Function: KCCQ2 questionnaire, 6-min walk test (6MWT) prior to and during an LVAD wean interval; Daily activity data collected throughout the study using a wearable biosensor (Actigraph) Cohort 1 (N=6) INXN-4001 80mg/ 40mL Cohort 2 (N=6) INXN-4001 80mg/ 80mL DSMB Review Study Design Distance walked by patients ON LVAD support during 6MWT at baseline (n=9) and after 6 months from RCSI procedure (n=4) Six Minute Walk Test: Distance Walked on LVAD No product-related adverse events observed to date LVEF for patients (A) ON LVAD support at baseline (n=11) vs. 6 months post RCSI (n=3) and (B) for patients OFF LVAD support after 6MWT at baseline (n=10) and after 6 months from RCSI (n=3) Left Ventricular Ejection Fraction (LVEF) 1RCSI: Retrograde Coronary Sinus Infusion 2KCCQ: The Kansas City Cardiomyopathy Questionnaire 3LVAD: Left Ventricular Assist Device

Precigen in 2019: Demonstrated achievement of milestones þ þ þ þ þ ‡ ‡Wholly owned subsidiary Precigen, Inc. will be renamed in connection with parent company name change. Initiate PRGN-3006 UltraCAR-T TM Phase 1 trial in AML and MDS Rapidly advance PRGN-3005 UltraCAR-T TM for solid tumor Rapidly advance PRGN-2009 AdenoVerse TM immunotherapy for solid tumor Rapidly advance one infectious disease candidate Rapidly advance preclinical candidates to go/no go

Capital allocation priorities for 2020

Precigen in 2020: Multiple upcoming clinical milestones for value creation Initial data from IP arm of PRGN-3005 UltraCAR-T TM Phase 1 trial in Ovarian Cancer Initial data from PRGN-3006 UltraCAR-T TM Phase 1 trial in AML and MDS Interim data from Phase 2 trial of AG013 in Oral Mucositis Interim data from Phase 1b/2a trial of AG019 in Type 1 Diabetes Phase 1 data completion of INXN-4001 in Heart Failure patients with LVAD Initiate Phase 1 trial of PRGN-2009 off-the-shelf AdenoVerse TM immunotherapy in HPV + cancers

Precigen in 2020: Upcoming preclinical milestones to drive value creation in long-term Rapidly advance PRGN-2011 AdenoVerse™ cytokine therapy towards Phase 1 study Advance next generation UltraCAR-T ™ candidate in IND-enabling studies Advance autoimmune disease candidate in IND-enabling studies Advance infectious disease candidate in IND-enabling studies Advance PRGN-5001 Multifunctional Therapeutic in IND-enabling studies

Precigen: World-class platform of innovative technologies and focused pipeline of precision medicines A focused healthcare company Advancing a robust portfolio of clinical and preclinical therapies Strong balance sheet combined with fiscal discipline Multiple value creating opportunities upcoming

