Precigen Announces Positive Phase 1 Data for PRGN-3005 Autologous UltraCAR-T® Cells Manufactured Overnight for Infusion Next Day to Advanced Stage Platinum Resistant Ovarian Cancer Patients
– PRGN-3005 was well-tolerated with no dose limiting toxicities, no CRS greater than Grade 2, and no neurotoxicity –
– PRGN-3005 cells demonstrated expansion and persistence when delivered via either intraperitoneal or intravenous infusion without lymphodepletion or via intravenous infusion after lymphodepletion demonstrating the effectiveness of mbIL15 –
– A single intravenous infusion following lymphodepletion decreased tumor burden in 67% of the heavily pretreated patients (median of 8 or more prior therapies) with 90% of individual target lesions showing stable disease or partial response –
– PRGN-3005 UltraCAR-T cells are being evaluated in the Phase 1b dose expansion study with intravenous infusion following lymphodepletion and incorporating repeat infusion –
– Best responder achieved stable disease for more than 18 months after failing 9 prior lines of treatment; results achieved with two doses of UltraCAR-T cells in the low millions –
"We are pleased with the results of the Phase 1 study which demonstrate a favorable safety profile for PRGN-3005 UltraCAR-T. Our UltraCAR-T therapies continue to be well-tolerated with no dose limiting toxicities across our clinical stage UltraCAR-T portfolio," said
"Ovarian cancer symptoms are not always obvious and it is frequently diagnosed at an advanced stage where cancer has spread to distant parts of the body, which results in historically poor outcomes," said
PRGN-3005 UltraCAR-T is an autologous chimeric antigen receptor T (CAR-T) cell therapy manufactured using non-viral gene delivery and is under investigation for the treatment of patients with advanced, recurrent platinum resistant ovarian, fallopian tube or primary peritoneal cancer.
PRGN-3005 utilizes
Conducted in collaboration with the
Patient Demographics
The study population includes patients with advanced stage (III/IV) recurrent ovarian, fallopian tube, and primary peritoneal cancer
Table 1: Phase 1 Dose Escalation Patient Demographics
|
IP (N=12) |
IV (N=6) |
IV with LD (N=9) |
||||
|
Age (years) |
||||||
|
Mean, Median |
57.4, 59.5 |
67.3, 69.5 |
62.7, 64 |
|||
|
Range |
38-70 |
54-76 |
47-77 |
|||
|
ECOG Status |
||||||
|
0 |
4 (33.3 %) |
3 (50 %) |
5 (55.6 %) |
|||
|
1 |
8 (66.7 %) |
3 (50 %) |
4 (44.4 %) |
|||
|
Baseline Tumor Burden (mm) |
||||||
|
Mean, Median |
71.2, 54.3 |
61.6, 46 |
97.1, 109.5 |
|||
|
Range |
15.2- 142.8 |
33.7 -145.7 |
44.5 - 149.8 |
|||
|
Baseline CA 125 |
||||||
|
Mean, Median |
1845.2, 1208.5 |
789.2, 199.5 |
539.3, 486 |
|||
|
Range |
82-8148 |
42-3035 |
60-1301 |
|||
|
Prior lines of therapy |
||||||
|
Mean, median (range) |
8.2, 8.5 (5-11) |
7.7, 8 (4-10) |
7.7, 8 (5-11) |
|||
|
4-5 |
2 (16.7 %) |
1 (16.7 %) |
2 (22.2 %) |
|||
|
6-11 |
10 (83.3 %) |
5 (83.3 %) |
7 (77.8 %) |
|||
Safety Data
PRGN-3005 was well-tolerated with low incidence of treatment related adverse events (TRAEs), no dose limiting toxicities (DLTs), and no neurotoxicity (Table 2). The most common side effects for the IV and IP arms without lymphodepletion were abdominal pain, fever and decreased absolute lymphocyte count (ALC). Serious Adverse Events included five incidences of Cytokine Release Syndrome (CRS), with no incidence of CRS greater than Grade 2. One patient with CRS required specific intervention which resolved following standard CRS management after 24 hours. There was no use of tocilizumab or dexamethasone or kill switch.
Table 2: PRGN-3005 Treatment Related Adverse Events
|
IP (N=12) |
IV (N=6) |
IV with LD (N=9) |
|||||
|
# of |
Patients (n, %) |
# of |
Patients (n, %) |
# of |
Patients (n, %) |
||
|
Abdominal Pain |
7 |
1 (8 %) |
0 |
0 |
0 |
0 |
|
|
Cytokine Release Syndrome |
0 |
0 |
1 |
1 (17 %) |
5 |
5 (56 %) |
|
|
Grade 1 CRS |
0 |
0 |
1 |
1 / 1 |
4 |
4 / 5 |
|
|
Grade 2 CRS |
0 |
0 |
0 |
0 / 1 |
1 |
1 / 5 |
|
|
Fever |
1 |
1 (8 %) |
1 |
1 (17 %) |
2 |
2 (22 %) |
|
|
Hypotension |
0 |
0 |
0 |
0 |
1 |
1 (11 %) |
|
|
ALC, Decreased |
6 |
2 (17 %) |
12 |
2 (33 %) |
0 |
0 |
|
|
Nausea |
0 |
0 |
0 |
0 |
1 |
1 (11 %) |
|
|
Small Intestinal Obstruction |
1 |
1 (8 %) |
0 |
0 |
0 |
0 |
|
|
White Blood Cell Decreased |
0 |
0 |
1 |
1 (17 %) |
1 |
1 (11 %) |
|
|
Summary of Events of Interest |
|||||||
|
DLTs |
0 |
0 |
0 |
0 |
0 |
0 |
|
|
Serious Adverse Events |
6 |
5 (41 %) |
2 |
1 (17 %) |
4 |
4 (44 %) |
|
|
Neurotoxicity |
0 |
0 |
0 |
0 |
0 |
0 |
|
Clinical Activity
Expansion Kinetics
PRGN-3005 administered either intraperitoneally or intravenously at doses as low as 2 million CAR-T cells resulted in a dose-dependent expansion and encouraging persistence in peripheral blood.
Tumor Responses
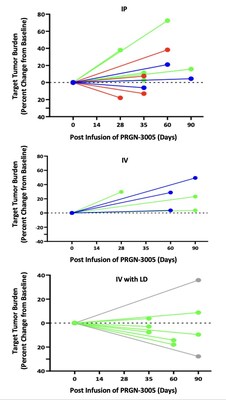
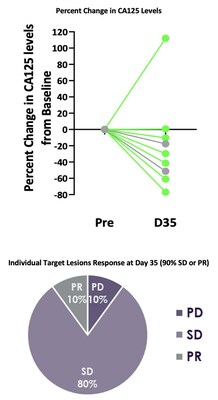
Best responses in patients treated without lymphodepletion were stable disease with complete responses in individual target lesions. Incorporating lymphodepletion prior to IV infusion led to an encouraging efficacy signal as demonstrated by a decrease in tumor burden in 67% (6/9) of patients (Figure 1), with concurrent decreases in CA125 at Day 35 in 89% (8/9) of patients and stable or partial response in 90% of the individual target lesions (Figure 2).
Figure 1: Target Tumor Burden
Figure 2: CA125 Levels and Target Lesion Response in the
Case Study
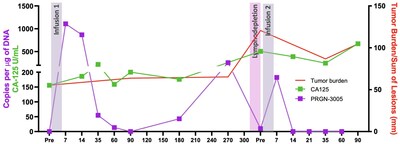
The best response was observed in a 73 year old female patient
Figure 3: Repeat Dosing and Tumor Response for Best Responder
Figure 4: Expansion and Tumor Burden/Sum of Lesions for Best Responder
PRGN-3005 UltraCAR-T cells are currently being evaluated in the Phase 1b dose expansion study at Dose Level 3 via IV infusion with lymphodepletion and incorporating repeat dosing.
About Ovarian Cancer
Worldwide, nearly 300,000 women are diagnosed with ovarian cancer every year1 with approximately 22,000 of them in the US2. Since early ovarian cancer is often without obvious symptoms, the disease is frequently diagnosed at an advanced stage where cancer has spread to distant parts of the body, such as the liver or lungs2,3. Five-year survival rates depend on stage and type of ovarian cancer with rates decreasing for advanced stage cancers that have spread to distant parts of the body3.
UltraCAR-T®
UltraCAR-T is a multigenic autologous CAR-T platform that utilizes
UltraCAR-T® Clinical Program
The UltraCAR-T platform has shifted the autologous CAR-T manufacturing paradigm using an advanced non-viral multigene delivery system and an overnight, decentralized manufacturing process for administration of autologous CAR-T cells one day after gene transfer to reduce vein-to-vein time.
UltraCAR-T® Library Approach
UltraPorator®
The UltraPorator system is an exclusive device and proprietary software solution for the scale-up of rapid and cost-effective manufacturing of UltraCAR-T therapies and potentially represents a major advancement over current electroporation devices by significantly reducing the processing time and contamination risk. The UltraPorator device is a high-throughput, semi-closed electroporation system for modifying T cells using
Trademarks
Cautionary Statement Regarding Forward-Looking Statements
Some of the statements made in this press release are forward-looking statements. These forward-looking statements are based upon the Company's current expectations and projections about future events and generally relate to plans, objectives, and expectations for the development of the Company's business, including the timing and progress of preclinical studies, clinical trials, discovery programs and related milestones, the promise of the Company's portfolio of therapies, and in particular its CAR-T and AdenoVerse therapies. Although management believes that the plans and objectives reflected in or suggested by these forward-looking statements are reasonable, all forward-looking statements involve risks and uncertainties, including the possibility that the timeline for the Company's clinical trials might be impacted by the COVID-19 pandemic, and actual future results may be materially different from the plans, objectives and expectations expressed in this press release. The Company has no obligation to provide any updates to these forward-looking statements even if its expectations change. All forward-looking statements are expressly qualified in their entirety by this cautionary statement. For further information on potential risks and uncertainties, and other important factors, any of which could cause the Company's actual results to differ from those contained in the forward-looking statements, see the section entitled "Risk Factors" in the Company's most recent Annual Report on Form 10-K and subsequent reports filed with the
References
1
2American Cancer Society Ovarian Cancer Special Section. Accessed via ACS website.
3
Investor Contact:
Vice President, Investor Relations
Tel: +1 (301) 556-9850
investors@precigen.com
Media Contacts:
press@precigen.com
glenn.silver@finnpartners.com
![]() View original content to download multimedia:https://www.prnewswire.com/news-releases/precigen-announces-positive-phase-1-data-for-prgn-3005-autologous-ultracar-t-cells-manufactured-overnight-for-infusion-next-day-to-advanced-stage-platinum-resistant-ovarian-cancer-patients-301842691.html
View original content to download multimedia:https://www.prnewswire.com/news-releases/precigen-announces-positive-phase-1-data-for-prgn-3005-autologous-ultracar-t-cells-manufactured-overnight-for-infusion-next-day-to-advanced-stage-platinum-resistant-ovarian-cancer-patients-301842691.html
SOURCE