Precigen Announces Positive Phase 1 Data for Off-the-Shelf PRGN-2009 AdenoVerse™ Immunotherapy Alone and in Combination with an Investigational Checkpoint Inhibitor in Patients with Recurrent/Metastatic HPV-associated Cancers
– PRGN-2009 combined with an investigational checkpoint inhibitor resulted in a 30% ORR in patients with heavily pre-treated HPV-associated cancers that were naïve or resistant to checkpoint blockade with prolonged duration of responses –
– Recurrent/metastatic HPV-associated cancers (cervical, anal, oropharyngeal, etc.) are incurable by current therapies –
– PRGN-2009 was safe and well-tolerated with only Grade 1 or 2 treatment related adverse events –
– PRGN-2009 treatment induced HPV-specific T-cell immune responses and subsequently enhanced T-cell responses with repeat administrations without the development of neutralizing antibodies in the majority of patients –
"Recurrent/metastatic HPV-associated cancers such as cervical, anal, and oropharyngeal are incurable by current therapies and there remains significant unmet need for new safe and effective treatments. Checkpoint inhibitors alone have shown some promise, but have not produced durable responses and many patients relapse or become resistant," said
PRGN-2009 is an
The Phase 1 trial is an open label, single-center study evaluating safety and response of PRGN-2009 as a monotherapy (Arm A) and in combination with bintrafusp alfa (Arm B) in previously treated adult patients with R/M HPV-associated cancers. In the monotherapy arm, patients (N = 6) enrolled in two sequential PRGN-2009 dose level cohorts, Dose Level 1 (1x1011 particle units (PU)) and Dose Level 2 (5x1011 PU) delivered via subcutaneous injection. In the combination arm, PRGN-2009 was administered at the recommended phase 2 dose (RP2D) in combination with bintrafusp alfa.
The primary objective of the study was to evaluate safety and RP2D of PRGN-2009 and safety of PRGN-2009 in combination with a checkpoint inhibitor. Secondary objectives included Objective Response Rate (ORR) (per RECIST 1.1), and progression free survival (PFS).
Patient Characteristics
Seventeen adult patients were enrolled in the Phase 1 study (Table 1). Patients received up to 20 doses of PRGN-2009 for a duration of 1.8 to 17.9 months in the monotherapy arm and 0.5 to 23.0 months in the combination arm. The median age in both arms was 61. The median number of prior lines of therapies in the metastatic setting was 2.5 for the monotherapy arm and 2 for the combination arm. All patients in the monotherapy arm (N=6) and 10 of 11 patients in the combination arm received prior immune checkpoint blockade (ICB) therapy.
|
Table 1. Patient Demographics and Clinical Characteristics |
||||
|
Monotherapy Arm (N=6) |
Combination Arm (N=11) |
|||
|
Age, years (median, range) |
61 (43-70) |
61 (54-80) |
||
|
Female, n (%) |
6 (100) |
3 (27) |
||
|
Tumor types (n,%) Oropharyngeal Cervical Anal Vaginal |
- 3 (50.0) 2 (33.3) 1 (16.7) |
7 (63.6) 3 (27.3) 1 (9.1) - |
||
|
HPV status (n, %) HPV16 HPV18 Other N/A |
3 (50) - 2 (33.3) 1 (16.7) |
9 (81.8) 1 (9.1) 1 (9.1) - |
||
|
Previous lines of therapy in metastatic setting, median (range) |
2.5 (2-3) |
2 (1-4) |
||
|
ICB exposure, n (%) Primary resistance Secondary resistance |
6 (100) 4 (66.7) 2 (33.3) |
10 (90.9) 5 (50) 5 (50) |
||
Safety Data
PRGN-2009 treatment in both monotherapy and combination arms was safe and well-tolerated (Table 2). In both study arms, there was a low incidence of treatment-related adverse events (TRAEs) with only Grade 1 or 2 TRAEs in the monotherapy arm. The most common TRAEs in the monotherapy arm were injection site reactions, flu-like symptoms, fatigue and rash. In addition to these in the combination arm, patients also experienced Grade 1 or 2 epistaxis, headache, keratoacanthoma, fever, decreased lymphocyte count, anemia and oral hemorrhage. TRAEs reported in the combination arm were in line with the safety profile reported for bintrafusp alfa and only Grade 1 or 2 TRAEs were attributable to PRGN-2009 in the combination arm.
|
Table 2: Safety Data |
||||||||
|
Monotherapy Arm (N=6) |
Combination Arm (N=11) |
|||||||
|
Treatment-related adverse events, n (%) |
Grade 1-2 (all) |
Grade 3-4 (all) |
Grade 1-2 (≥10%) |
Grade 3-4 (all) |
||||
|
Injection site reactions |
4 (66.7) |
0 |
9 (81.8) |
0 |
||||
|
Flu-like symptoms, |
3 (50.0) |
0 |
6 (54.5) |
0 |
||||
|
Fatigue |
2 (33.3) |
0 |
3 (27.3) |
0 |
||||
|
Rash, maculopapular |
1 (16.7) |
0 |
3 (27.3) |
0 |
||||
|
Epistaxis |
0 |
0 |
3 (27.3) * |
0 |
||||
|
Headache |
0 |
0 |
3 (27.3) |
0 |
||||
|
Keratoacanthoma |
0 |
0 |
3 (27.3)* |
0 |
||||
|
Fever |
0 |
0 |
2 (18.2) |
0 |
||||
|
Lymphocyte count, decreased |
0 |
0 |
2 (18.2)* |
0 |
||||
|
Anemia |
0 |
0 |
2 (18.2)* |
0 |
||||
|
Oral hemorrhage |
0 |
0 |
2 (18.2)* |
0 |
||||
|
Duodenal Hemorrhage |
0 |
0 |
0 |
2 (18.2)*†‡ |
||||
|
Pharyngeal mucositis |
0 |
0 |
0 |
1 (9.1) * |
||||
|
*Attributed to bintrafusp alfa; † both patients concurrently receiving NSAIDs; ‡ 1 patient died following refusal of standard supportive medical management measures (blood transfusion). |
Clinical Activity
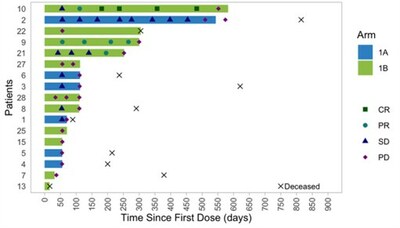
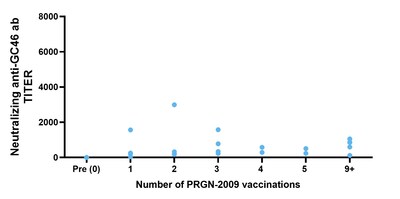
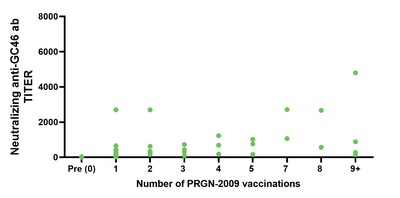
Tumor responses were observed in patients after treatment with PRGN-2009 in combination with bintrafusp alfa (Arm 1B), including in ICB-resistant patients (Table 3). PRGN-2009 combined with bintrafusp alfa resulted in a 30% ORR in patients with pretreated R/M HPV-associated cancers with prolonged duration of responses (Table 3, Figure 1). The majority of patients developed HPV-16 and/or HPV-18 specific immune responses after treatment with PRGN-2009 in both monotherapy and combination arms (Table 4) without the development of neutralizing antibodies (Figure 2).
|
Table 3: Best Response by Arm |
||||
|
Monotherapy Arm |
Combination Arm |
|||
|
Evaluable patients, n |
6 |
10 |
||
|
Best response, n |
||||
|
CR |
- |
1a |
||
|
PR |
- |
2c,d |
||
|
SD |
4b |
1 |
||
|
PD |
2 |
6 |
||
|
ORR, % (95% CI) |
- |
30 (6.7-65.3) |
||
|
a immune checkpoint blockade (ICB)-resistant; b 1 SD confirmed; c 1 PR confirmed; d 1 ICB-resistant, 1 TCR treatment-resistant; 2 patients treated beyond progression without delayed response; CI: confidence interval |
|
Figure 1: Time to Response and Duration of Response to Treatment |
|
Table 4: HPV-specific T-cell Immune Responses |
||
|
Monotherapy Arm |
Combination Arm |
|
|
HPV-16, n/N (%) |
5/6 (83) |
7/10 (70) |
|
HPV-18, n/N (%) |
5/6 (83) |
7/8 (88) |
|
HPV-16 and/or HPV-18 n/N (%) |
6/6 (100) |
8/10 (80) |
|
N: number of patients tested |
||
|
Figure 2: Neutralizing Antibodies |
Phase 2 Clinical Study in Newly Diagnosed Oropharyngeal Squamous Cell Carcinoma
The Phase 2 portion of the study is ongoing at the RP2D and enrollment was completed in the monotherapy arm with 20 evaluable patients with newly diagnosed oropharyngeal squamous cell carcinoma (OPSCC). An interim clinical data presentation from the Phase 2 monotherapy arm is expected in the second half of 2023.
Phase 2 Randomized Control Study in Recurrent or Metastatic Cervical Cancer
The Company recently announced that the
###
AdenoVerse™ Immunotherapy
AdenoVerse™ Immunotherapy Clinical Program
For patients interested in enrolling in NCI-led clinical studies, please call NCI's toll-free number 1-800-4-Cancer (1-800-422-6237) (TTY: 1-800-332-8615), email NCIMO_Referrals@mail.nih.gov, and/or visit the website: https://trials.cancer.gov.
Trademarks
Cautionary Statement Regarding Forward-Looking Statements
Some of the statements made in this press release are forward-looking statements. These forward-looking statements are based upon the Company's current expectations and projections about future events and generally relate to plans, objectives, and expectations for the development of the Company's business, including the timing and progress of preclinical studies, clinical trials, discovery programs and related milestones, the promise of the Company's portfolio of therapies, and in particular its CAR-T and AdenoVerse therapies. Although management believes that the plans and objectives reflected in or suggested by these forward-looking statements are reasonable, all forward-looking statements involve risks and uncertainties, including the possibility that the timeline for the Company's clinical trials might be impacted by the COVID-19 pandemic, and actual future results may be materially different from the plans, objectives and expectations expressed in this press release. The Company has no obligation to provide any updates to these forward-looking statements even if its expectations change. All forward-looking statements are expressly qualified in their entirety by this cautionary statement. For further information on potential risks and uncertainties, and other important factors, any of which could cause the Company's actual results to differ from those contained in the forward-looking statements, see the section entitled "Risk Factors" in the Company's most recent Annual Report on Form 10-K and subsequent reports filed with the
Investor Contact:
Steven M. Harasym
Vice President, Investor Relations
Tel: +1 (301) 556-9850
investors@precigen.com
Media Contacts:
Donelle M. Gregory
press@precigen.com
Glenn Silver
Lazar-FINN Partners
glenn.silver@finnpartners.com
![]() View original content to download multimedia:https://www.prnewswire.com/news-releases/precigen-announces-positive-phase-1-data-for-off-the-shelf-prgn-2009-adenoverse-immunotherapy-alone-and-in-combination-with-an-investigational-checkpoint-inhibitor-in-patients-with-recurrentmetastatic-hpv-associated-cancers-301841791.html
View original content to download multimedia:https://www.prnewswire.com/news-releases/precigen-announces-positive-phase-1-data-for-off-the-shelf-prgn-2009-adenoverse-immunotherapy-alone-and-in-combination-with-an-investigational-checkpoint-inhibitor-in-patients-with-recurrentmetastatic-hpv-associated-cancers-301841791.html
SOURCE