Precigen Presents New Data Supporting the Safety, Clinical Activity, Expansion and Persistence of PRGN-3006 UltraCAR-T® at the 62nd ASH Annual Meeting and Exposition
AML is a rapidly progressing disease with poor prognosis and high unmet need.
An investigator-initiated, non-randomized Phase 1/1b dose-escalation study to evaluate the safety and maximal tolerated dose of PRGN-3006 UltraCAR-T is currently ongoing in collaboration with the
Key findings:
- At the data cutoff (
November 10 ): - Six patients have been treated across the two lowest dose levels in Cohort 1 (no lymphodepletion):
- N=3 at Dose Level 1 (3 x 104 - ≤ 1 x 105 UltraCAR-T cells/kg); Total 1.8 to 7 x 106 UltraCAR-T cells
- N=3 at Dose Level 2 (1 x 105 - ≤ 3 x 105 UltraCAR-T cells/kg); Total 24 to 29 x 106 UltraCAR-T cells
- Three patients have been treated at the lowest dose level in Cohort 2 (with lymphodepletion):
- N=3 at Dose Level 1 (3 x 104 - ≤ 1 x 105 UltraCAR-T cells/kg); Total 4.9 x 106 to 1 x 107 UltraCAR-T cells
- Encouraging expansion and persistence of PRGN-3006 UltraCAR-T was observed in both lymphodepletion and non-lymphodepletion cohorts and across all dose levels.
- PRGN-3006 has been safe and well-tolerated with no dose limiting toxicities (DLTs), no neurotoxicity, and a low incidence of treatment-related adverse events (TRAEs) and serious adverse events (SAEs). A few treatment-related SAEs have been observed, including transient grade 1-3 cytokine release syndrome (CRS), which is more indicative of the biologic activity of the cells.
- There has been a 100% manufacturing success rate using the UltraCAR-T manufacturing process.
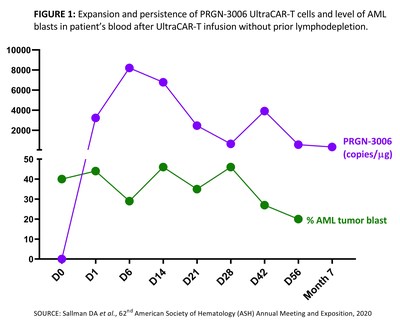
A case study of the patient with the longest follow-up as of the data cutoff was also presented. This patient received, one day after gene transfer and without prior lymphodepletion, a very low dose, approximately three hundred thousand UltraCAR-T per kilogram (3 x 105 UltraCAR-T/kg) for a total of only 24 million UltraCAR-T. She is a 69 year old female with secondary AML (sAML) and four prior lines of therapy, including induction chemotherapy (IC), allogenic hematopoietic stem cell transplantation (allo-HSCT), HMA plus venetoclax (HMA+VEN), refractory to all therapy post allo-HSCT. The patient had approximately 40% peripheral blasts and 47% bone marrow blasts at baseline.
Case study findings:
- After a very low dose infusion without prior lymphodepletion, PRGN-3006 UltraCAR-T cells demonstrated robust expansion and persistence in blood at seven months post-infusion at the time of the most recent sample collection (see FIGURE 1).
- UltraCAR-T cells demonstrated trafficking to bone marrow and the ability to expand and persist in bone marrow.
- The patient showed a decline in blast levels in blood and bone marrow concomitant with UltraCAR-T expansion and persistence (see FIGURE 1) and had stable disease. Patient follow-up is ongoing.
"There is an urgent need for novel therapies for relapsed or refractory AML patients as the median overall survival for this patient population is less than six months. Current CAR-T approaches for AML have faced challenges due to long manufacturing durations resulting in subsequent delays in treatment," said
"Currently commercialized CAR-T therapies have not demonstrated the persistence needed to drive sustained, durable responses," said
About Acute Myeloid Leukemia (AML)
AML is a cancer that starts in the bone marrow, but most often moves into the blood.1 Though considered rare, AML is among the most common types of leukemia in adults.2 In 2019, it was estimated that 21,450 new cases of AML would be diagnosed in the US.2 AML is uncommon before the age of 45 and the average age of diagnosis is about 68.2 The prognosis for patients with AML is poor with an average 5–year survival rate of approximately 25 percent overall, and less than a 5 percent 5–year survival rate for patients older than 65.3 Amongst elderly AML patients (≥ 65 years of age), median survival is short, ranging from 3.5 months for patients 65 to 74 years of age to 1.4 months for patients ≥ 85 years of age.3
About Myelodysplastic Syndrome (MDS)
MDS are diseases of the bone marrow generally found in adults in their 70s.4 Incidence in the US is not known for sure, but estimates range from 10,000 each year and higher.4 Using International Prognostic Scoring System (IPSS-R), median survival for MDS patients can vary from less than one year for the "very high" IPSS-R risk group to more than eight years for the "very low" IPSS-R group.4
About PRGN-3006 UltraCAR-T
PRGN-3006 UltraCAR-T is a multigenic autologous CAR-T cell treatment utilizing
Trademarks
Cautionary Statement Regarding Forward-Looking Statements
Some of the statements made in this press release are forward-looking statements. These forward-looking statements are based upon the Company's current expectations and projections about future events and generally relate to plans, objectives, and expectations for the development of the Company's business, including the timing and progress of preclinical studies, clinical trials, discovery programs and related milestones, the promise of the Company's portfolio of therapies, and in particular its CAR-T therapies, and the Company's refocus to a healthcare-oriented business. Although management believes that the plans and objectives reflected in or suggested by these forward-looking statements are reasonable, all forward-looking statements involve risks and uncertainties, including the possibility that the timeline for the Company's clinical trials might be impacted by the COVID-19 pandemic, and actual future results may be materially different from the plans, objectives and expectations expressed in this press release. The Company has no obligation to provide any updates to these forward-looking statements even if its expectations change. All forward-looking statements are expressly qualified in their entirety by this cautionary statement. For further information on potential risks and uncertainties, and other important factors, any of which could cause the Company's actual results to differ from those contained in the forward-looking statements, see the section entitled "Risk Factors" in the Company's most recent Annual Report on Form 10-K and subsequent reports filed with the
References
1
2
3 Thein, M., et al., Outcome of older patients with acute myeloid leukemia: an analysis of SEER data over 3 decades. Cancer, 2013. 119(15): p.2720-7
4
For more information, contact:
|
Investor Contact: Vice President, Investor Relations Tel: +1 (301) 556-9850 |
Media Contact: |
![]() View original content to download multimedia:http://www.prnewswire.com/news-releases/precigen-presents-new-data-supporting-the-safety-clinical-activity-expansion-and-persistence-of--prgn-3006-ultracar-t-at-the-62nd-ash-annual-meeting-and-exposition-301186957.html
View original content to download multimedia:http://www.prnewswire.com/news-releases/precigen-presents-new-data-supporting-the-safety-clinical-activity-expansion-and-persistence-of--prgn-3006-ultracar-t-at-the-62nd-ash-annual-meeting-and-exposition-301186957.html
SOURCE